 Toxicity of Carbon Dioxide Gas Exposure, CO2 Poisoning Symptoms, Carbon Dioxide Exposure Limits
Toxicity of Carbon Dioxide Gas Exposure, CO2 Poisoning Symptoms, Carbon Dioxide Exposure Limits
- POST a QUESTION or COMMENT about Carbon Dioxide CO2 gas levels indoors, CO2outside, CO2testing, CO2poisoning symptoms, & CO2 exposure limits and toxicity for humans
Carbon Dioxide CO2 Exposure Limits & Toxicity to humans:
This article series discusses normal and abnormal CO2 gas levels, the toxicity and exposure limits for exposure to carbon dioxide gas (CO2). We discuss Carbon Dioxide gas levels in outdoor air, in buildings, typical CO2 levels and conditions under which levels are unsafe.
We discuss the symptoms of carbon dioxide poisoning, describe different types of risks where high levels of CO2 may be present, and present data about the effects of CO2 exposure. Seek prompt advice from your doctor or health/safety experts if you have any reason to be concerned about exposure to toxic gases. Links on this page also direct the reader to carbon monoxide gas information in a separate document.
We give references and explanation regarding toxicity of Carbon Dioxide. Links on this page also direct the reader to carbon monoxide gas information in a separate document.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
Information about Concentrations of Carbon Dioxide - CO2 in Air
 - Daniel Friedman, with special thanks to Per Levéen, Telia Mobile, Sweden [1], Dr. Roy Jensen, (Canada) [2]. and Stephen Fisher,
B.Sc., Sales Director,
K.D.Fisher & Company, Pty., Ltd., Australia, [3] for technical editing & comments.
- Daniel Friedman, with special thanks to Per Levéen, Telia Mobile, Sweden [1], Dr. Roy Jensen, (Canada) [2]. and Stephen Fisher,
B.Sc., Sales Director,
K.D.Fisher & Company, Pty., Ltd., Australia, [3] for technical editing & comments.
Article Series Contents
The Effects of CO2 at Various Levels or Concentrations in Air
Recent research suggests that even at modest elevations of CO2 levels indoors mental acuity and decision making ability may be impaired. In turn that research suggests that improved building fresh-air ventilation beyond contemporary IAQ standards (5-10 cfm of fresh air per person 13 per minute) may not be adequate.
This table describes what happens to a typical occupant's mental and physical health as the concentrations of carbon dioxide increase in the air a person is breathing.
Carbon Dioxide Concentrations vs. Health Effects & IAQ |
|||
| CO2 ppm | CO2 Percent of Air | Duration | Effects |
| 350 - 400 ppm | 0.035% - 0.04% [12] | Typical outdoor CO2 level - 0.3% by volume = 0.23 mmHg | |
| 600 | 0.06% | hours | reduced mental performance for some [8] |
| 1,000 | 0.10% | hours | reduced test scores [7] common in classrooms [9], |
| 1,200 | 0.12% | hours | Indoors indicates poor fresh-air ventilation [6], |
| 1,000 - 2000 | 0.10% - 0.20% | hours | Occupant complaints of drowsiness, poor indoor air [14] |
| 2,500 | 0.25% | hours | Significantly reduced mental performance for some [7] |
| 3,000 | 0.3% | 6+ hrs. | Perceived reduced IAQ w/ bioeffluents present [10] |
| 2000 - 5000 | 0.20% - 0.5% | hours | Headaches, sleepiness, and stagnant, stale, stuffy air. Poor concentration, loss of attention, increased heart rate, slight nausea [14] |
| 5,000 | 0.5% | hours | Reduced mental performance, found in some classrooms [8] Toxicity or oxygen deprivation may occur [14] This is the PEL for daily workplace exposures. |
| 10,000 | 1% | minutes + | Drowsiness [1] |
| 12,000 | 1.2% | ? | Headache complaints 9 mmHg [12] |
| 20,000 | 2% | min. to hrs. | Awareness of poor IAQ [2] |
| Above 20,000 | > 2% | min. to hrs. | Heaviness in chest, difficulty breathing, possible acidosis after several hours [3] |
| 30,000 | 3% | minutes | Breathing rate doubles [4] |
| 40,000 | 4% | minutes | Immediately harmful due to oxygen deprivation [14] |
| 50,000 | 5% | minutes | Breathing rate 4 x normal, threshold of toxicity [4] |
| Above 20,000 | > 5% | minutes | Toxic, unconsciousness, death [5] |
Notes to the table above
Watch out: IF YOU SUSPECT ANY BUILDING GAS-RELATED POISONING GO INTO FRESH AIR IMMEDIATELY and get others out of the building, then call your fire department or emergency services for help.
1,000,000 ppm of a gas = 100 % concentration of the gas, and 10,000 ppm of a gas in air = a 1% concentration.
- At 1% concentration of carbon dioxide CO2 (10,000 parts per million or ppm) and under continuous exposure at that level, such as in an auditorium filled with occupants and poor fresh air ventilation, some occupants are likely to feel drowsy.
At lower CO2 levels we may be seeing effects of a reduction in the relative amount of oxygen rather than direct toxicity of CO2 - The concentration of carbon dioxide must be over about 2% (20,000 ppm) before most people are aware of its presence unless the odor of an associated material (auto exhaust or fermenting yeast, for instance) is present at lower concentrations.
- Above 2% concentration in air, carbon dioxide may cause a feeling of heaviness in the chest and/or more frequent and deeper respirations. If exposure continues at that level for several hours, minimal "acidosis" (an acid condition of the blood) may occur but more frequently is absent.
- Breathing rate doubles at 3% CO2 and is four times the normal rate at 5% CO2.
Symptoms of high or prolonged exposure to carbon dioxide include headache, increased heart rate, dizziness, fatigue, rapid breathing, visual and hearing dysfunctions. Exposure to higher levels may cause unconsciousness or death within minutes of exposure. - Toxic levels of carbon dioxide: at levels above 5%, concentration CO2 is directly toxic.
- Indoor CO2 levels of 1,200 ppm and above indicate poor fresh-air ventilation. Respiratory or IAQ complaints at this indoor CO2 level may be due to increased concentrations of other offgassing products from building materials (formaldehyde) or increased odor levels from various sources.
- Some studies suggest worse test scores at CO2 level of 1000, and much worse test score performance at an indoor CO2 level of 2,500 ppm than at 600 ppm (Satish 201, Liu 2017, Allen 2015, )
- 600 ppm CO2 concentration levels indoors is common in crowded spaces and may result in reduced mental performance on some tasks for some people (Fisk 2013)
- Miller, Sherry ... NYT in process U Colorado
- ... moderate concentrations of bioeffluents, but not pure CO2 , will result in deleterious effects on occupants during typical indoor exposures. (Zhang 2015)
However Liu found that 3000 ppm CO2 did not exacerbate IAQ complaints at elevated temperatures up to 35°C (95°F)(Liu 2017) - NASA's SMAC for CO2 was 5000 but this control was deleted in 2017 (NASA 2017)
- (Law 2010 citing (Sliwka 1998))
- ASHRAE Standard 6.2.1, Table 6.2.2.1 provides recommended outdoor or "fresh air" ventilation rates expressed in cfm/person or L/s/person as well as rates expressed in cfm/ft2 or l/s-m2 . These rates vary by type of occupancy category. For example break rooms need 5 cfm/person while a media center or science lab needs 10 cfm/person.
ASHRAE 6.2.1 VENTILATION for ACCEPTABLE INDOOR AIR QUALITY [PDF] - Wisconsin DHS, Health Effects of Carbon Dioxide, Chemical reference number (CAS): 124-38-9 - (2021) - Wisconsin Department of Health Services, 1 West Wilson Street
Madison, WI 53703
USA, Tel: 608-266-1865 - retrieved 2022/02/25, original source, https://www.dhs.wisconsin.gov/chemical/carbondioxide.htm
Excerpt:
Exposure to CO2 can produce a variety of health effects. These may include headaches, dizziness, restlessness, a tingling or pins or needles feeling, difficulty breathing, sweating, tiredness, increased heart rate, elevated blood pressure, coma, asphyxia, and convulsions. The levels of CO2 in the air and potential health problems are given in the table above, . [Adapted from this and other sources - Ed.] - CARBON DIOXIDE HEALTH EFFECTS [PDF] U.S. EPA, retrieved 2022/07/15 original source: https://www.epa.gov/sites/default/files/2015-06/documents/co2appendixb.pdf
Excerpt:
Appendix B presents an overview of the acute health effects associated with carbon dioxide. Part I discusses the dangerous, lethal effects of carbon dioxide at high exposure concentrations. The minimum design concentration of carbon dioxide for a total flooding system is 34 percent (340,000 ppm).
When used at this design concentration, carbon dioxide is lethal. Part II discusses the potentially beneficial effects of carbon dioxide at low exposure concentrations and the use of added carbon dioxide in specialized flooding systems using inert gases. - CARBON DIOXIDE CO2: GEOLOGIC SEQUESTRATION HEALTH EFFECTS: "VULNERABILITY EVALUATION FRAMEWORK FOR GEOLOGIC SEQUESTRATION OF CARBON DIOXIDE [PDF] (2008) US EPA, EPA430-R-08-009, July 2008, web search August 2010
- CARBON DIOXIDE CO2: GEOLOGIC SEQUESTRATION [PDF], U.S EPA, web search 08/28/2010, original source:
http://www.epa.gov/climatechange/emissions/co2_gs_tech.html
How to Convert mmHg to ppm concentrations of a gas in air
On Earth, the ambient CO2 concentration is about 0.03% by volume (0.23 mm Hg). (Law 2010)
Watch out: These example calculations converting ppm to mmHg are subject to technical review
Conversion factor for concentration of a gas in air in ppm to concentration in mmHg
mmHg = ppm / 1,304.34
Derivation:
0.03% concentration of CO2 = 300 ppm = 0.23 mmHg (Law 2010)
300 ppm / 0.23 mmHg = 1,304.34
Examples:
5000 ppm / 1,304.34 = 3.8 mmHg
0.5% concentration of CO2 = 5000 ppm CO2 = 3.8 mmHg [ w/o adjustments for temperature, humidity, etc.] - Ed.
To Convert ppm to mmHg
divide ppm by 1,304.34 to get mmHg
or because 1/1,304.34 = 0.007666713 you can also
multiply ppm x 0.00076667 to get mmHg
...
To Convert mmHg to ppm
divide mmHg by 0.00076667 to get ppm
or
multiply mmHg by 1,304.35 to get ppm
Also see Lenntech on converting ppm at https://www.lenntech.com/calculators/ppm/converter-parts-per-million.htm
Also see mmHg(CO2) = mmHg(system) * (reading from sensor in ppm) / 1,000,000 cited at https://forums.parallax.com/discussion/147975/converting-co2-ppm-to-mmhg
Quoting
So if you had standard pressure then the mmHg of "system" would be 760mmHg.
So in this case:
mmHg(CO2) = (reading from sensor in ppm) * 0.000760
Edit: The above will only work if the parts per million is a mole fraction (same as volume fraction in many cases). If the measurement is ppm mass fraction, then you'd need and additional conversion factor.
See also the scientific explanation at Botero, Nick, How to Calculate the PPM From Vapor Pressure (2017), https://sciencing.com/calculate-ppm-vapor-pressure-6457861.html
What are the Typical CO2 Carbon Dioxide Levels Found in Outdoor Air?
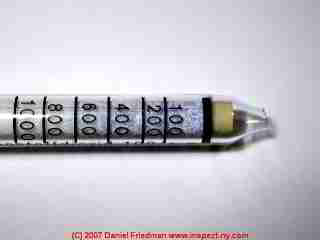
 The photo shows a Drager colorimetric gas detection tube used to test the CO2 levels in air. In an indoor air test (in our laboratory) the detector found that the CO2 level was about 600ppm which is typical of indoor air and is considered an acceptable and safe level.
The photo shows a Drager colorimetric gas detection tube used to test the CO2 levels in air. In an indoor air test (in our laboratory) the detector found that the CO2 level was about 600ppm which is typical of indoor air and is considered an acceptable and safe level.
While authorities indicate that CO2 is present in outdoor air at 0.035% [12], (or 0.04% in popular sources - 2021 - Ed.) our own measurements [DF] indicate that at a given locality the actual CO2 level varies according to local conditions including temporal factors such as nearby fossil-fuel engines such as automobiles & buses.
In our measurements outdoors the typical carbon dioxide CO2 level in air typically varies between 300 ppm to 400 ppm.
400 ppm is a 0.04% concentration of a gas in air - slightly higher than the "official" figure.
Comparing Outdoor CO2 Levels with Indoor CO2 in Buildings
When studying carbon dioxide levels inside of a building we therefore start with an outdoor baseline measurement, or several, obtained at varying distances from the structure.
A comparison of the actual outdoor CO2 level with even a relatively low level of indoor CO2 (600 ppm and higher) may indicate a lack of adequate fresh air entering a building.
Carbon dioxide gas level measurements may be used in a study of indoor air quality even when the absolute levels of CO2 itself are not harmful. But as we explain below, at higher levels CO2 itself can affect building occupants and can even become dangerous or fatal.
Distinguishing between high carbon dioxide levels CO2 and low oxygen levels O2 in air
What may be unclear in some cases is whether the sub-acute (sub-toxic) effects at modestly-elevated levels of CO2 in air stem from more from exposure to higher levels of carbon dioxide or whether they are due to reduced levels of oxygen.
In an enclosed space such as a tight home or an enclosed basement or work space, increasing the level of CO2 is likely to simultaneously reduce the proportion of Oxygen (O2) in that same breathing air.
Some experts opine that complaints that seem to be associated with high CO2 problem in many if not most circumstances are likely to be actually due to the corresponding reduction in available oxygen in air rather than high toxicity levels of CO2 in the air.
As carbon dioxide levels climb above a few percent the relative proportions of gases making up that air change: the concentration of oxygen in the air inhaled is reduced as the amount of CO2 is increased.
However, the TOXIC effects of elevated levels of CO2 are serious at levels when the oxygen reduction effects are only minor. [3]
CO2 Carbon Dioxide poisoning symptoms
IF YOU SUSPECT ANY BUILDING GAS-RELATED POISONING GO INTO FRESH AIR IMMEDIATELY and get others out of the building, then call your fire department or emergency services for help.
Here we discuss Carbon Dioxide gas levels in outdoor air, in buildings, typical CO2 levels and conditions under which levels are unsafe. We discuss the symptoms of carbon dioxide poisoning, describe different types of risks where high levels of CO2 may be present, and present data about the effects of CO2 exposure.
Seek prompt advice from your doctor or health/safety experts if you have any reason to be concerned about exposure to toxic gases. Links on this page also direct the reader to carbon monoxide gas information in a separate document.
High CO2 Exposure Case Reports
Case Report Example of Drowsiness Possibly Ascribe to Elevated Levels of Carbon Dioxide (CO2) in Vassar Temple, Poughkeepsie
Ca. 1990 and using the Draeger (Dräger) gas detection tubes and a simple hand pump - shown here, the author [DF] performed a series of carbon dioxide level measurements during religious services at the Vassar Temple, a Reform Jewish Synagogue in Poughkeepsie, New York.
A previous inspection of the building's heating and air conditioning system had revealed that there was no working provision for the introduction of fresh outdoor air or "make-up air" into the buildings's duct system.
Carbon Dioxide levels were measured (discreetly) from a central first floor position in a pew during the Yom Kippur religious services, at ten minute intervals during this high holy day service.
For readers un-familiar with this religious service we include this explanation:
Synagogue is a critical part of Yom Kippur, offering five prayer services. During each one, the congregation confesses its sins collectively. Some attendees wear white clothing or a kittel, a white garment that symbolizes a burial shroud, the clothing of angels, and the purity of forgiveness. - (Blakemore 2021)
- Prior measurements had confirmed that when the sanctuary was empty or occupied by just a few people, the CO2 level in the synagogue air typically varied between 300 ppm to 400 ppm.
- During Yom Kippur it is common for the synagogue sanctuary to be filled to or near capacity.
As the religious service progressed we observed the CO2 level increasing steadily from its starting point to 1,000 ppm, then onwards to 1,850 ppm.
At this point the author observed a number of attendees appearing to be dozing.
Expert sources point out that as the CO2 level in a building exceeds 1000 ppm and approaches 2000 ppm there may be occupant complaints of drowsiness and poor air, and indeed we observed some worshippers appearing to nod off. Or perhaps they were simply contemplating their sins and thinking about starting the new year afresh - with sins forgiven and maybe afresh with better air.
- Following the service the author discussed his findings with the Rabbi, Steven Arnold. We joked that perhaps the Rabbi had worried that his sermon, coming late in the service, was perhaps not as stimulating as he might have liked, but new evidence of elevated CO2 level in the Vassar Temple Synagogue argued that the real culprit might be poor indoor air quality.
- The Rabbi Arnold noted that except during the high holy days religious service attendance was perhaps so low that elevated levels of CO2 in th building never occurred, so attendees dozed less and sermons seemed more invigorating.
- We recommended that the outdoor makeup air system be repaired and returned to service.
Research
- EFFECTS of CO2 at VARIOUS CONCENTRATIONS
- Erin Blakemore, "Why Yom Kippur is the holiest day of the Jewish Year", National Geographic, 24 September 2021. - retrieved 2022/02/25, original source, https://www.nationalgeographic.com/history/article/yom-kippur-history-traditions
Case Report Example of Fatal Levels of Carbon Dioxide (CO2) in a Building
This is important because we recently had an accident with CO2 in Sweden killing two persons.
According to the newspapers CO2 is nontoxic and it is the decreased oxygen levels that kills. THIS CONCEPT IS WRONG.
Using the calculation equation above one can quickly conclude that adding 31 litres of CO2 to a 100 litre enclosed space would result in a 23.7% CO2 by volume in air, which would be almost instantly fatal, and 16% oxygen by volume in air, (equivalent to breathing at 2800 meters above sea level, which is dangerous, because it can lead to poor decision making, but not fatal).
It is the toxic properties of CO2 that is fatal, not the drop in oxygen.
In conclusion, in the event of a CO2 Cylinder leak, it is the toxic properties of CO2 that is fatal, long before oxygen levels have been reduced to fatally low levels.
According to the calculation given below, a level of 1.4% CO2 cause a drop of oxygen from 20.9% to 19.5%. As the arithmetic above shows, This calculation is misleading. Saying that adding 1.4% CO2 causes oxygen to drop to 20.9 - 1.4 = 19.5% is like saying that adding 20.9% CO2 would cause oxygen to drop to 20.9 - 20.9 = 0% That is of course not true. The correct and more precise calculation is provided above this paragraph.
Reduced % Oxygen & Other Gases When Carbon Dioxide (CO2) Reaches 5% of room air
Using either of the above calculation methods, the introduction of 5% CO2 to the room's air volume will reduce each of the components of normal air to 95% of their original proportion.
Component of Air |
Standard Air Percentage | After Addition of 5% CO2 |
Nitrogen (N2) |
78.0% | 74.1% |
Oxygen (O2) |
20.9% | 19.85% |
Argon (Ar) |
1.0% | 0.95% |
Carbon Dioxide (CO2) |
0.04% | 5.04% |
Notes to the table above
Jensen [2] at REFERENCES notes that if we increase the CO2 level in air in an enclosed space from its normal level of about 0.03% (we counted it as starting at 0) to a level of 1.4%, we obtain a corresponding decrease in the oxygen level from its normal level (at sea level) of about 20.9% down to 19.5%, for a 6.7% reduction in the amount of oxygen available.
The amount of oxygen lost is 6.7 % (1.4/20.9 * 100 %). Our earlier version of this document was incorrect in this calculation.
A 5% level of CO2 is directly toxic, yet as can be seen, the Oxygen level is at 19.85%, whereas the standard Australian 1st alarm level is set at 19.5%, so an oxygen sensor would not reach an alert level, despite serious CO2 levels being reached.
Example of Fatal Levels of CO2 Carbon Dioxide in a Building
Per Levéen has thoughtfully provided the detailed analysis comparing the hazards of elevated carbon dioxide in a building with the accompanying reduction of oxygen (O2 ) in the same space if the percentage of CO2 is increased from a leak from a CO2 gas cylinder. [1]
The data following has been modified by Stephen Fisher, B.Sc, Dip. Ed., Sales Director of K.D.Fisher & Co., Pty. Ltd.[3]
FACT: 100 liters of air contains:
- 20.9 liters of oxygen (20.9%)
- 0.04 liters of CO2 (0.04%)
Preliminary Assumptions: If the 100 litres is contained in a balloon like membrane, then 1.4 litres of gas can be added, at the same pressure.
If we add 1.4 litres of CO2 to this mixture, we will get 101.4 litres of air which has an elevated CO2 content, and a reduced oxygen content, see the calculation immediately below:
Carbon Dioxide: (1.4 + 0.04) / 101.4 = 0.014 = 1.42 % CO2 by volume in air
This is is an increase in CO2 percentage of 35.5 times above the “normal level” of 0.04% CO2 by volume in air. 1.42% CO2 can also be expressed as 14,000ppm (parts per million) CO2.
Oxygen: 20.9 / 101.4 = 0.206 = 20.6 % oxygen.
This is a reduction in the oxygen percentage of 0.3% by volume in air, below the “normal” 20.9% Oxygen by volume in air.
This change in the mix of gases in air when the level of CO2 increased results in a decrease with 1.4% in the oxygen level (and not 6.7% as was stated
at EXAMPLE of Reduced Oxygen Level in a Building)
However, KC Baczewski PE writes that the above calculation should be
((1.4/100)*20.9) = 0.29 %. i.e. a reduction in the “normal” oxygen level of 0.29%
You displace O2 and N2 for a final composition of:
- N2 (Nitrogen) = (79.1/100)*(100-1.4) = 77.99%
- O2 (Oxygen) = (20.9/100)*(100-1.4) = 20.61%
- Ar (Argon) = 1%
- CO2 (Carbon Dioxide) = 1.4%
Total = 100.00%.
Stephen Fisher agrees with this calculation method, which would involve extracting 1.4 litres of air, prior to the addition of the 1.4 litres of CO2 to the remaining 98.6 litres, which restores the total volume to 100 litres.
This calculation has the advantage of providing a standard 100 litres of “air”, and consequently the number of litres of each component corresponds to the % by volume of each component gas of the mixture.[3]
Example of Reduced Oxygen Level in a Building
According to EXAMPLE of FATAL LEVELS of CO2 CARBON DIOXIDE in a BUILDING, the math of the following example is not quite correct.
We have kept Dr. Jensen's comments (below) but they should be read together with the detailed example and calculation provided above by Per Levéen.
More carbon dioxide may mean less oxygen: Let's say, sake of simplicity, that we're converting oxygen to carbon dioxide in an enclosed space. Then when the CO2 level has increased from its normal amount in air (about 0.03%) up to a higher concentration in air of 1.4% CO2 the concentration of oxygen in air will have decreased from 20.9 to 19.5%.
Reducing the oxygen concentration from 20.9% down to 19.5% is equal to a 6.7% reduction in the oxygen level. -- Thanks to thanks to Dr. Roy Jensen for assistance with these details.
What are the effects on humans (and other animals) of reduction of the oxygen levels in air?
At sea level, breathing air in which the O2 level has fallen to 16% percent is equivalent to being at the top of a 9,200-foot mountain - close to the level at which many people will experience shortness of breath while walking.
12% Oxygen in air at sea level corresponds to breathing normal air at an elevation of about 17,400 feet.
Document notes:
Original content, since extensively edited and expanded by several experts, this article began in 1985 with a literature search & search on Compuserve's Safety Forum by Dan Friedman. This is background information, obtained from expert sources. However information presented here may be incomplete.
Special thank-you to Thomas K. Keenan + Puschkinallee 6C + 12435 Berlin + F.R.G., for careful reading and editing assistance 29 November 2020.
...
Continue reading at CO2 EXPOSURE LIMITS or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see CARBON DIOXIDE - CO2 FAQs - questions & answers posted originally at this page.
Or see these
Carbon Dioxide Articles
- AIR QUALITY on COMMERCIAL AIRCRAFT
- CARBON DIOXIDE - CO2
- CARBON MONOXIDE - CO - a different gas
- CONCENTRATIONS of GASES in AIR
- FRESH AIR VENTILATION RATES & STANDARDS
- GAS DETECTION INSTRUMENTS
- GAS EXPOSURE EFFECTS, TOXIC
- INDOOR AIR QUALITY IMPROVEMENT GUIDE
Suggested citation for this web page
CARBON DIOXIDE - CO2 at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to GAS HAZARDS in BUILDINGS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
Our Comment Box is provided by Countable Web Productions countable.ca
Citations & References
In addition to any citations in the article above, a full list is available on request.
- Allen, Joseph G., Piers MacNaughton, Usha Satish, Suresh Santanam, Jose Vallarino, and John D. Spengler. "ASSOCIATIONS OF COGNITIVE FUNCTION SCORES WITH CARBON DIOXIDE, VENTILATION, AND VOLATILE ORGANIC COMPOUND EXPOSURES IN OFFICE WORKERS: A CONTROLLED EXPOSURE STUDY OF GREEN AND CONVENTIONAL OFFICE ENVIRONMENTS." Environmental health perspectives 124, no. 6 (2015): 805-812. Retrieved 2019/05/12, original source: https://ehp.niehs.nih.gov/doi/full/10.1289/ehp.1510037
Objective: We simulated indoor environmental quality (IEQ) conditions in “Green” and “Conventional” buildings and evaluated the impacts on an objective measure of human performance: higher-order cognitive function.
Methods: Twenty-four participants spent 6 full work days (0900–1700 hours) in an environmentally controlled office space, blinded to test conditions. On different days, they were exposed to IEQ conditions representative of Conventional [high concentrations of volatile organic compounds (VOCs)] and Green (low concentrations of VOCs) office buildings in the United States. Additional conditions simulated a Green building with a high outdoor air ventilation rate (labeled Green+) and artificially elevated carbon dioxide (CO2) levels independent of ventilation.
Results: On average, cognitive scores were 61% higher on the Green building day and 101% higher on the two Green+ building days than on the Conventional building day (p < 0.0001). VOCs and CO2 were independently associated with cognitive scores.
Conclusions: Cognitive function scores were significantly better under Green+ building conditions than in the Conventional building conditions for all nine functional domains. These findings have wide-ranging implications because this study was designed to reflect conditions that are commonly encountered every day in many indoor environments - Bakó-Biró, Zs, Derek J. Clements-Croome, Neena Kochhar, Hazim B. Awbi, and Marilyn J. Williams. "Ventilation rates in schools and pupils’ performance." Building and Environment 48 (2012): 215-223.
Abstract:
This paper is a development of our earlier work [5], [6], [11]. The effects of classroom ventilation on pupils’ performance were investigated in 8 primary schools in England.
In each school the concentrations of carbon dioxide and other parameters were monitored for three weeks in two selected classrooms. In 16 classrooms interventions were made to improve the ventilation rate and maintain the temperature within an acceptable range using a purpose-built portable mechanical ventilation system.
As a result of the interventions the provision of outdoor air to the classrooms was improved from the prevailing levels of about 1 l/s per person to about 8 l/s per person.
The pupils and teachers in the classrooms studied were usually exposed to unacceptably poor air quality conditions, with CO2 concentrations of up to 5000 ppm, much higher than the average recommended levels of 1500 ppm and the preferred level of 1000 ppm.
The results of computerized performance tasks performed by more than 200 pupils showed significantly faster and more accurate responses for Choice Reaction (by 2.2%), Colour Word Vigilance (by 2.7%), Picture Memory (by 8%) and Word Recognition (by 15%) at the higher ventilation rates compared with the low ventilation conditions.
The present investigation provides strong evidence that low ventilation rates in classrooms significantly reduce pupils’ attention and vigilance, and negatively affect memory and concentration. The physical environment therefore affects teaching and learning.
Excerpt: Increased levels of CO2 from a mean of 690 ppm to a mean of 2909 ppm lead to a detriment in power of attention of about 5%.". Satish et al - Dooley et al, GTSP, 2006: Carbon Dioxide Capture and Geologic Storage: A Core Element of a A Global Energy Technology Strategy to Address Climate Change (PDF, 37 pp., 6.05 MB, About PDF). April 2006, JJ Dooley et al. Global Energy Technology Strategy Program (GSTP)
- [9] Bruggeman et al. 2007 Acid-base balance in chicken embryois...incubated under high CO2 concentrations... Respiratory Physiology and Neurobiology 159:147-154
- [11] Bar-Ilan, Amir and Jacob Marder, Adaptations to Hypercapnic conditions in the Nutria..., Comp. Biochem. Physiol. Vol 75A, No 4, pp 603-608, 1983
- [12] CCOHS "Health Effects of Carbon Dioxide Gas", CCOHS, Canadian Centre for Occupational Health and Safety, web search 02/15/2012, original source: http://www.ccohs.ca/oshanswers/chemicals/chem_profiles/carbon_dioxide/health_cd.html
- [10] De Smit et al, 2006 Emryonic developmental plasticity of the chick: Increased CO2 ... Comparative Biochemistry and Physiology, Part A 145: 166-175
- [3] Fisher, Stephen, Australian and New Zealand Sales Director, K.D.Fisher & Company, Pty., Ltd. 18 Benjamin Street, St. Mary's, Adelaide, South Australia, 5042, Australia.
Ph: (08) 8277-3288 (Int): +61-8-8277-3288 Fax: (08) 8276-4024 (Int): +61-8-8276-4024 E-mail: stevef@kdfisher.com.au [by email, Feb 2012] website: http://www.kdfisher.com.au/ Quoting from the company's website
KD Fisher & Co. Pty. Ltd., Safety and Welfare: OHS & W training facilities located on premises; Gas detection monitoring & consultation; Safety & Security products; Electric components, Power & switchgear products, Electrical/Electronic service & engineering
Mr. Fisher adds "Our company is family owned, and employs 30 personnel, and has been specifying, and designing gas detection systems, using "bought in" detectors from overseas manufacturers, and developing sampling systems to allow the most proficient system for many applications, including jet fuel leakage detection systems for military aircraft hangars, and tanker parking shelters, for the Australian Dept. of Defence. "
- [3-a] Fisher, Stephen, "DANGERS OF CARBON DIOXIDE, HEALTH EFFECTS OF CARBON DIOXIDE GAS" [PDF], K.D. Fisher & Co. Pty, Ltd., 18 Benjamn St., St. Marys, Adelaide, South Australia 5042, website: www.kdfisher.com.au, Tel: 08-8277-3288. Offices in Sydney & Melbourne. This document consists of selected reproductions from the CCOHS (Canada's National Occupational Health & Safety Resource) with minor Australian applications & modifications, and cites the InspectAPedia carbon dioxide gas hazards article found on this page.
- Fisk, William J., Usha Satish, Mark J. Mendell, Toshifumi Hotchi, and Douglas Sullivan. IS CO2 AN INDOOR POLLUTANT? HIGHER LEVELS OF CO2 MAY DIMINISH DECISION MAKING PERFORMANCE [PDF] ASHRAE JOURNAL 55, no. LBNL-6148E (2013). Retrieved 2019/05/13, original source: https://www.osti.gov/servlets/purl/1171812
- Forrester, Mathias B. Poison Control News Dry Ice: A Potential Halloween Hazard. [PDF] Texas Public Health Journal: 4.
- Gamble, J.L. (ed.), K.L. Ebi, F.G. Sussman, T.J. Wilbanks, (Authors), ANALYSES OF THE EFFECTS OF GLOBAL CHANGE ON HUMAN HEALTH AND WELFARE AND HUMAN SYSTEMS. A Report by the U.S. Climate Change Science Program and the Subcommittee on Global Change Research. [PDF] CCSP, 2008: U.S. Environmental Protection Agency, Washington, DC, USA. Web search 08/28/2010, original source: http://nepis.epa.gov/
- Gonzales, Lorenzo, Sateesh Sakhamuri, Surujpal Teelucksingh, and Ronan G. Ali. "Dry ice (solid carbon dioxide) exposure with disastrous consequences." QJM: An International Journal of Medicine 110, no. 11 (2017): 757-758. - retrieved 2022/02.25 original source: https://academic.oup.com/qjmed/article/110/11/757/3979423?login=true
Excerpt: A 62-year-old female was evaluated for sudden onset of cough, dyspnoea and lightheadedness thought to be cardiac in origin. Symptoms started acutely while driving her vehicle transporting ice cream on dry ice in unsealed containers. The windows were closed with the air conditioning on.
After 15 min, she began experiencing dyspnoea, cough and light-headedness. She attempted to drive toward the motorway shoulder but then had no further memory of events. She awoke some 30 min later more than 5 km away in her overturned vehicle. She later learned that she had struck several vehicles before overturning at the side of a major roadway (Figure 1A and 1B). Her rescuer reported that she was unconscious with her eyes rolling upwards and frothing at her mouth. She awoke shortly after the rescuer broke the car window to extricate her from the vehicle.
She had no obvious injuries, was in no pain and had no recollection of the preceding 30 min. She had no history of significant illness and never used tobacco, alcohol or illicit drugs. Evaluation in the Emergency Department (ED) revealed normal physical signs, normal imaging of chest and brain and normal routine blood investigations. An elevated troponin level at that time invoked suspicion of acute coronary syndrome. Cardiac workup including echocardiography, stress testing and Holter monitoring excluded myocardial ischemia and pathological arrhythmias. - [8] Holloway and Heath, 1984 Ventilatory Changes in the Golden Hamster..., Laboratory Rat...., Comp. Biochem. Physiol., Vol. 77A, No 2, pp. 267-273
- James, John T. "Surprising effects of CO2 exposure on decision making." In 43rd international conference on environmental systems, p. 3463. 2013. https://doi.org/10.2514/6.2013-3463 or https://arc.aiaa.org/doi/pdf/10.2514/6.2013-3463
Abstract: Carbon dioxide (CO2 ) is released from humans while they live and work in spacecraft or spacesuites. Removal of this anthropogenic pollutant requires major resources, which increase dramatically as the permissible levels of CO2 set to protect human health and performance are reduced.
The current Spacecraft Maximum Allowable Concentration (SMAC) of CO2 aboard the ISS is 5.3 mmHg; however, according to Chits (mission action requests), NASA and its international partners havce agreed to control CO2 levels to less than 4 mmHg.
In the meantime, retrospective investigations attempting to associate crew symptoms with elevated CO2 levels over the life of the International Space Station (ISS) are underway to determine if this level is sufficient to protect against health and performance decrements.
Anecdotal reports suggest that crewmembers are not able to perform complex tasks as readily in spaceflight as they were able to during ground-based training. Recently the effects of CO2 on decision making have been investigated. Using data from this one study, we show that there are obvious adverse effects of CO2 exposures on decision making above 1.5 mmHg.
The impications and limitations of this study are paramount in determining future CO2 SMACs for human spaceflight, both aboard the ISS and in exploration-class missions. - James, John T., PhD., & Ariel Macatangay, PhD., CARBON DIOXIDE - OUR COMMON "ENEMY" [PDF], NASA/Johnson Space Center, Houston TX USA (2009) retrieved 2019/05/13 original source https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20090029352.pdf
Abstract Exerpt: We discuss strategies for highly-efficient, regenerable removal of CO2 that could meet the 1000-day SMAC of 0.5%, which would apply to long-duration voyages to Mars
Note: as of 2017 the NASA SMAC for CO2 had been deleted - Ed.
Carbon dioxide (CO2) SMACs have been deleted – CO2 does not fit SMAC paradigm and is being managed based on expected performance and health decrements and the associated risks. NASA Standard 3001 is currently under revision to provide guidance on acceptable CO2 levels. - NASA JSC 20584, September 2017 cited at NASA in this list.
- [2]
Jensen, Roy, PhD, Department of Chemistry, Grant MacEwan College, Edmonton, AB for technical review and critique 8/23/07.
Dr. Jensen notes that if we increase the CO2 level in air in an enclosed space from its normal level of about 0.03% (we counted it as starting at 0) to a level of 1.4%, we obtain a corresponding decrease in the oxygen level from its normal level (at sea level) of about 20.9% down to 19.5%, for a 6.7% reduction in the amount of oxygen available.
The amount of oxygen lost is 6.7 % (1.4/20.9 * 100 %). Our earlier version of this document was incorrect in this calculation. - [6] Klemens C. Baczewski PE, email correspondence, 4/29/2009 discussed correct CO2 calculations.
- Law, Jennifer, Sharmi Watkins, and David Alexander. IN-FLIGHT CARBON DIOXIDE EXPOSURES AND RELATED SYMPTOMS: ASSOCIATION, SUSCEPTIBILITY, AND OPERATIONAL IMPLICATIONS. [PDF] NASA Center for AeroSpace Information, 7115 Standard Drive, Hanover MD 21076 USA Tel: 301-621-0390, NASA Technical Paper 216126 (2010).
Excerpt: On Earth, the ambient CO2 concentration is about 0.03% by volume (0.23 mm Hg). - Langford, Nigel J. "Carbon dioxide poisoning." Toxicological reviews 24, no. 4 (2005): 229-235.
- Liu, Weiwei, Weidi Zhong, and Pawel Wargocki. IS CO2 AN INDOOR POLLUTANT? DIRECT EFFECTS OF LOW-TO-MODERATE CO2 CONCENTRATIONS ON HUMAN DECISION-MAKING PERFORMANCE. [PDF] Building and Environment 114 (2017): 96-105.
- Liu, Weiwei, Weidi Zhong, and Pawel Wargocki. "Performance, acute health symptoms and physiological responses during exposure to high air temperature and carbon dioxide concentration." Building and Environment 114 (2017): 96-105.
Abstract
Human subjects were exposed for 3 h in a climate chamber to the air temperature of 35 °C that is an action level, at which the working time needs to be diminished in China.
The purpose was to put this action level to test by measuring physiological responses, subjective ratings and cognitive performance, and compare them with responses at temperature of 26 °C (reference exposure). Moreover, CO2 was increased to 3000 ppm (CO2 exposure) at 35 °C to further examine, whether this change will have any effect on the measured responses.
Compared with the reference exposure, exposure to 35 °C caused subjects to report feeling uncomfortably warm, to rate the air quality as worse, to report increased sleepiness and higher intensity of several acute health symptoms.
Eardrum temperature, skin temperature, heart rate and body weight loss all increased significantly at this exposure, arterial oxygen saturation decreased significantly, while the percentage of adjacent inter-beat cardiac intervals differing by > 50 m (pNN50) decreased significantly, indicating elevated stress.
The performance of addition and subtraction tasks decreased significantly during this exposure, as well.
Increasing CO2 to 3000 ppm at 35 °C caused no significant changes in responses. Present results reaffirm the selection of 35 °C as an action level, and show that concurrently occurring high CO2 levels should not exacerbate the hazards. - Metz, Bert, Davidson, Ogunlade, de Coninck, Heleen, Loos, Manuela, and Meyer, Leo (Eds.) IPCC, 2005: Special Report on Carbon Dioxide Capture and Storage, Special Report of the Intergovernmental Panel on Climate Change []. Cambridge University Press, The Edinburgh Building Shaftesbury Road, Cambridge CB2 2RU England
- NASA, SPACECRAFT MAXIMUM ALLOWABLE CONCENTRATIONS FOR AIRBORNE CONTAMINANTS [PDF] (2017), US NASA, Human Health and Performance Directorate
Toxicology Group
Environmental Sciences Branch
Biomedical Research and Environmental Sciences Division, JSC 20584, 09/2017, retrieved 2018/05/13 original source: https://www.nasa.gov/sites/default/files/atoms/files/jsc_20584_signed.pdf
Excerpt:
Carbon dioxide (CO2) SMACs have been deleted – CO2 does not fit SMAC paradigm and is being managed based on expected performance and health decrements and the associated risks. NASA Standard 3001 is currently under revision to provide guidance on acceptable CO2 levels.
Previously the NASA SMAC for CO2 aboard the ISS (International Space Station) was 5.3 mmHg also expressed in some documents as 5000 ppm. (James 2013) - Ed. - [5] OSHA, TABLE Z-1 LIMITS FOR AIR CONTAMINANTS, 1910.1000 TABLE Z-1 [PDF] OSHA standard for air contaminant limits (http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=9992) - includes for CO2, Carbon dioxide.........| CAS No. 124-38-9 | 5000 ppm | 9000 mg/m3 limits for carbon dioxide as an air contaminant.
- [1] Per Levéen, email comments 23 May 2009 . Mr. Levéen is with Telia, the leading mobile telephone operator in Sverige (Sweden). By telephone Telia (not Mr. Levéen) can be reached at 90 200 or From abroad at +46-771-99 02 00
- Romeo L, Prigioni P, Marcheselli S, Marchiori L, Cerpelloni M, Fiorini C, Brugnone F. Intossicazione acuta da anidride carbonica: descrizione di due casi mortali [Acute poisoning with carbon dioxide: report of 2 fatal cases]. Med Lav. 2002 Jan-Feb;93(1):26-33. Italian. PMID: 11987499.
Excerpt:
Conclusions: All these findings suggest that the atmosphere inside the concrete well was altered by the fermentative processes of pollina. The death of the two workers, caused by a poorly oxygenated atmosphere with a high concentration of carbon dioxide, can be classified under the confined space hypoxic syndrome (CSHS). - Satish, Usha, Mark J. Mendell, Krishnamurthy Shekhar, Toshifumi Hotchi, Douglas Sullivan, Siegfried Streufert, and William J. Fisk. "Is CO2 an indoor pollutant? Direct effects of low-to-moderate CO2 concentrations on human decision-making performance." Environmental health perspectives 120, no. 12 (2012): 1671-1677. Retrieved 2019/05/13, original source: https://ehp.niehs.nih.gov/doi/full/10.1289/ehp.1104789
Conclusions
Increases in indoor CO2 concentrations resulting from the injection of ultrapure CO2, with all other factors held constant, were associated with statistically significant and meaningful reductions in decision-making performance.
At 1,000 ppm CO2, compared with 600 ppm, performance was significantly diminished on six of nine metrics of decision-making performance.
At 2,500 ppm CO2, compared with 600 ppm, performance was significantly reduced in seven of nine metrics of performance, with percentile ranks for some performance metrics decreasing to levels associated with marginal or dysfunctional performance.
The direct impacts of CO2 on performance indicated by our findings may be economically important, may disadvantage some individuals, and may limit the extent to which outdoor air supply per person can be reduced in buildings to save energy. Confirmation of these findings is needed. - Sliwka U, Krasney JA, Simon SG, Schmidt P, Noth J. Effects of sustained low-level elevations of carbon
dioxide on cerebral blood flow and autoregulation of the intracerebral arteries in humans. Aviat
Space Environ Med. 1998 Mar;69(3):299-306. (published first 1996)
Abstract:
Cerebral blood flow velocity (CBFv) was measured by insonating the middle cerebral arteries of 4 subjects using a 2 Mhz transcranial Doppler. Ambient CO2 was elevated to 0.7% for 23 days in the first study and to 1.2% for 23 days in the same subjects in the second study.
By non-parametric testing CBFv was elevated significantly by +35% above pre-exposure levels during the first 1-3 days at both exposure levels after which CBFv progressively readjusted to pre-exposure levels. Despite similar CBFv responses, headache was only reported during the initial phase of exposure to 1.2% CO2. Vascular reactivity to CO2 assessed by rebreathing showed a similar pattern with the CBFv increases early in the exposures being greater than those elicited later. An increase in metabolic rate of the visual cortex was evoked by having the subjects open and close their eyes during a visual stimulus. Evoked CBFv responses measured in the posterior cerebral artery were also elevated in the first 1-3 days of both studies returning to pre-exposure levels as hypercapnia continued.
Cerebral vascular autoregulation assessed by raising head pressure during 10 deg head-down tilt both during the low-level exposures and during rebreathing was unaltered. There were no changes in the retinal microcirculation during serial fundoscopy studies. The time-dependent changes in CO2 vascular reactivity might be due either to retention of bicarbonate in brain extracellular fluid or to progressive increases in ventilation, or both. Cerebral vascular autoregulation appears preserved during chronic exposure to these levels of ambient CO2. - Sliwka, U., J. A. Krasney, S. G. Simon, and P. Schmidt. "Effects of sustained low-level elevations of carbon dioxide on cerebral blood flow and autoregulation of the intracerebral arteries in humans." (1996).
- [7] Taylor, Lewis G. and G. Oscar Kreutziger, The Gaseous Environment of the Chick Embryo in Relation to Its Development and Hatchability, 1968 (printout does not include the Journal)
- US EPA CARBON DIOXIDE CO2: GEOLOGIC SEQUESTRATION, [PDF] U.S EPA, web search 08/28/2010, original source: http://www.epa.gov/climatechange/emissions/CO2_gs_tech.html
- US EPA Health Effects of Carbon Dioxide - see "National Advisory Committee for Acute Exposure Guideline Levels (AEGLs) for Hazardous Substances; Proposed AEGL Values, Federal Register Document", http://www.epa.gov/EPA-TOX/2002/February/Day-15/t3774.htm note that these are proposed guidelines
- US EPA GREENHOUSE GAS OVERVIEW: CARBON DIOXIDE [PDF] U.S. EPA, web search 08/28/2010, original source:
http://www.epa.gov/climatechange/emissions/CO2.html - US EPA, Health Effects of Carbon Dioxide - see "National Advisory Committee for Acute Exposure Guideline Levels (AEGLs) for Hazardous Substances; Proposed AEGL Values, Federal Register Document", htp://www.epa.gov/EPA-TOX/2002/February/Day-15/t3774.htm note that these are proposed guidelines
- US EPA Carbon Dioxide CO2: Geologic Sequestration Health Effects: "VULNERABILITY EVALUATION FRAMEWORK FOR GEOLOGIC SEQUESTRATION OF CARBON DIOXIDE" [PDF] , US EPA, EPA430-R-08-009, July 2008, web search August 2010,original source: http://www.epa.gov/climatechange/emissions/downloads/VEF-Technical_Document_072408.pdf
- Zhang, Xiaojing, Pawel Wargocki, and Zhiwei Lian. "Physiological responses during exposure to carbon dioxide and bioeffluents at levels typically occurring indoors." Indoor Air 27, no. 1 (2017): 65-77.
- Zhang, Xiaojing, Pawel Wargocki, Zhiwei Lian, and Camilla Thyregod. "Effects of exposure to carbon dioxide and bioeffluents on perceived air quality, self‐assessed acute health symptoms, and cognitive performance." Indoor air 27, no. 1 (2017): 47-64.
Abstract: The purpose of this study was to examine the effects on humans of exposure to carbon dioxide (CO2) and bioeffluents. In three of the five exposures, the outdoor air supply rate was high enough to remove bioeffluents, resulting in a CO2 level of 500 ppm.
Chemically pure CO2 was added to this reference condition to create exposure conditions with CO2 at 1000 or 3000 ppm. In two further conditions, the outdoor air supply rate was restricted so that the bioeffluent CO2 reached 1000 or 3000 ppm. The same 25 subjects were exposed for 255 min to each condition. Subjective ratings, physiological responses, and cognitive performance were measured.
No statistically significant effects on perceived air quality, acute health symptoms, or cognitive performance were seen during exposures when CO2 was added.
Exposures to bioeffluents with CO2 at 3000 ppm reduced perceived air quality; increased the intensity of reported headache, fatigue, sleepiness, and difficulty in thinking clearly; and reduced speed of addition, the response time in a redirection task, and the number of correct links made in the cue‐utilization test.
This suggests that moderate concentrations of bioeffluents, but not pure CO2, will result in deleterious effects on occupants during typical indoor exposures. - In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.


