 Stoichiometric Combustion: Perfect Fuel Combustion for Gas or Oil Burners
Stoichiometric Combustion: Perfect Fuel Combustion for Gas or Oil Burners
- POST a QUESTION or COMMENT about heating oil or gas fuel combustion - perfect or stoichiometric combustion.
Definition & uses of stoichiometric combustion:
Here we discuss the optimal fuel combustion process, boiler or furnace efficiency, courtesy of aerospace engineer Herman Vogel how has helped by providing a definition of Stoichiometric Combustion.
We explain and give formulas for Theoretical Fuel to Air Ratios for Complete Combustion (Stoichiometric Combustion).
Stoichiometric Combustion discusses theoretical fuel to air ratios for hydrocarbon fuels (kerosene, jet fuel, heating oil, LP gas, etc) in which a fuel is burned completely, producing only carbon dioxide and water, with no other byproducts.
We discuss for both oil and gas burners, the characteristics of perfect combustion of fuel. This article is part of our online Guide to Oil Burners for heating systems, boilers & furnaces, including their basic parts, operation, maintenance, performance & money-saving tips.
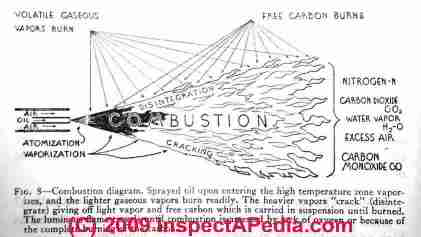
Our sketch (page top) shows how an oil burner gun atomizes and sprays heating oil into the combustion chamber - Audel Oil Burner Guide
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
Theoretical Fuel to Air Ratios for Complete Combustion (Stoichiometric Combustion)
 This article series answers most questions about central heating and water heating systems to aid in troubleshooting, inspection, diagnosis, and repairs.
This article series answers most questions about central heating and water heating systems to aid in troubleshooting, inspection, diagnosis, and repairs.
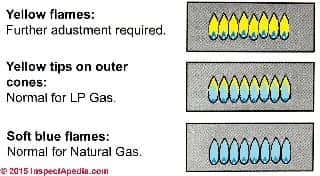
The gas flame illustration at left is explained in detail
at GAS BURNER FLAME & NOISE DEFECTS
This article (below) focuses on fossil fuel combustion efficiency using heating oil as an example.
Herman Vogel, Aerospace Engineer
In brief: Stoichiometric combustion is by thermodynamic definition the theoretical combustion of every drop of fuel when mixed with the correct amount of air (oxygen) to yield exhaust products of only CO2 and H2O.
Stoichiometric combustion is by thermodynamic definition the theoretical combustion of every drop of fuel when mixed with the correct amount of air (a basic mixture of oxygen and nitrogen gases) to yield exhaust products of only CO2 and H2O.
However, such combustion is ideal and in reality doesn't occur since the burning in furnaces, automobiles and jet-engines is always incomplete and less than 100% due to engineering design limitations.
Therefore while our two ideal exhaust products are relatively benign, non-stoichiometric combustion rules in the real world. This results in burning rich, as no matter how much extra air we cleverly add, it is never able to chemically react with all the fuel.
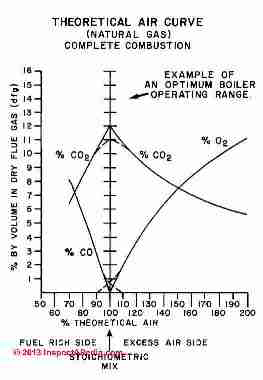 Hence the resultant unburned fuel gets exposed to the high combustion temperatures and chemically reacts to form additional exhaust products of CO (Carbon Monoxide) and NO (Nitric Oxide), which can be dangerous exhaust products to both people and the environment.
Hence the resultant unburned fuel gets exposed to the high combustion temperatures and chemically reacts to form additional exhaust products of CO (Carbon Monoxide) and NO (Nitric Oxide), which can be dangerous exhaust products to both people and the environment.
Stoichiometric combustion has a chemical reaction equation attached to it:
C12H26(l) + 37/2 O2(g) + 2(37) N2(g) → 12 CO2(g) + 13 H2(g) + 2(37) N2(g)
For the above chemical reaction to be complete, both sides of the equation (about the arrow) must have its chemical elements matched.
So, if the left-side has 12 Carbon molecules, then the right-side must also have 12.
Note that we are using air which contains a weighted ratio of N2/O2 = 2x37 / (37/2) = 4 (100% air = 80% N2 + 20% O2), or four times as much Nitrogen exists in a given volume of air as does Oxygen.
Also, the above chemical reaction equation represents kerosene fuel which is considered a Dodecanese (liquid) hydrocarbon having the chemical formula C12H26.
That means it contains a molecular composition of 12-carbon atoms and 26-hydrogen atoms and is a liquid at room temperature.
Kerosene is the main constituent of Jet Propulsion (JP) fuels, where other additives include various blends of differently distilled crude oils.
The fuel to air ratio by chemical weight of the above relationship is:
FAR = [mf_C12H26 / ma_O2+N2] = (12*12.011+26*1.008)/(37/2*32+2*37*28.014) = 0.064 or 6.4%
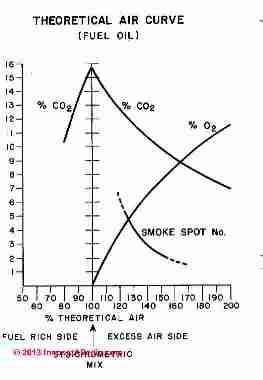 This says that for complete combustion of typical hydrocarbon fuels, and in particular kerosene, we need 6.4 lbs. of fuel for every 100 lbs. of air that we burn. Clearly, on a relative basis, we need a lot of air to completely burn our small amount of fuel.
This says that for complete combustion of typical hydrocarbon fuels, and in particular kerosene, we need 6.4 lbs. of fuel for every 100 lbs. of air that we burn. Clearly, on a relative basis, we need a lot of air to completely burn our small amount of fuel.
The beauty of the above chemical equation is that not only does it define the required fuel-to-air ratio, but it also provides us with:
- The expectant adiabatic (pure, no losses in heat) flame temperature released by this chemical reaction as based on chemical heats of formation (4,310 R), and
- The heating value released per pound of kerosene (18,950 BTU/lbs.) as based on its chemical formulation plus heat of formation.
Technical Note: The temperature above is in units of R (Rankin) not (F). If it were F (Fahrenheit) we have 4,310R - 460R = 3,850F.
What Does Complete Combustion (Stoichiometric Combustion) Mean to the Heating System Designer?
The above three pieces of information helps the furnace designer to properly size combustion chambers and their air-fans, and to burn the correct amount of fuel to keep a home comfortable.
It also helps the designer to choose the appropriate materials to avoid furnace melt-down. Note that the theoretical adiabatic flame temperature is very high.
While some of this temperature is reduced due to furnace heat losses, flame temperatures are generally controlled by intentionally burning lean.
This dramatically reduces the theoretical flame temperature based on the lean chemistry of combustion, plus additional temperature reductions are realized by diluting high temperatures using the extra cold air entering the combustion chamber.
So, the furnace designer has a whole arsenal of possibilities to work with in designing today's reliable fuel oil furnaces.
Heating Fuel Combustion Research regarding Complete Combustion & Stoichiometry
- Farías, Oscar, Françoise Jara, and Róbinson Betancourt. "Theoretical and experimental study of the natural draft in chimneys of buildings for domestic gas appliances." Energy and Buildings 40, no. 5 (2008): 756-762.
- Mueller, Charles J. The quantification of mixture stoichiometry when fuel molecules contain oxidizer elements or oxidizer molecules contain fuel elements. No. 2005-01-3705. SAE Technical Paper, 2005.
- Mueller, John C., STOICHIOMETRY: A TOOL IN GAS APPLIANCE DEVELOPMENT, [PDF] (1959) for a detailed exposition on stoichiometric combustion.
- Storms, E. K., and B. A. Mueller. "A study of surface stoichiometry and thermionic emission using LaB6." Journal of Applied Physics 50, no. 5 (1979): 3691-3698.
...
Continue reading at OIL BURNER INSPECTION & REPAIR or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
Suggested citation for this web page
COMPLETE COMBUSTION, STOICHIOMETRIC at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to HEATING SYSTEMS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
Our Comment Box is provided by Countable Web Productions countable.ca
Citations & References
In addition to any citations in the article above, a full list is available on request.
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.

